Combating Cockayne Syndrome
We are in the golden age of biotech, of smart, exciting advancements in gene replacement therapy that have the potential to save our precious and beloved children's lives. Our time is now. Let's get to work.
Riaan Research Initiative seeks to identify, promote, and/or fund cutting-edge, intelligent, innovative, and thoughtful research proposals aimed at aggressively and effectively translating therapies from the bench to the bedside, including the use of N-of-1 trial models. Such therapies may include gene therapy or editing or drug repurposing screens.
Upcoming Study
Seeking a Clinician to Assess GDF15 Levels in Cockayne Syndrome Patients
 Riaan Research Initiative is seeking a clinical partner to conduct a small and short study involving analyzing serum samples from children with Cockayne Syndrome. The study’s goal is to assess GDF-15 serum levels in a small number of children with Cockayne Syndrome and determine whether the levels are elevated, and if so, how elevated. This study is based on a recent paper that hypothesizes that GDF-15, a growth differentiation factor that tells the brain to reduce appetite and induces weight loss, may be perpetually released in Cockayne Syndrome children, causing adverse effects.
Researchers from Dr. KJ Patel’s lab discovered that when formaldehyde is introduced to the body, it causes DNA damage. The kidneys subsequently release GDF-15, which triggers appetite suppression while the body attempts to repair the DNA damage. This response is also commonly seen in patients undergoing chemotherapy. Scientists believe that the body may have developed this evolutionary appetite block to prevent a person from ingesting more toxins while it is repairing DNA damage. Researchers theorize that as DNA damage builds up in Cockayne Syndrome patients, GDF-15 continues to be released because the DNA damage is never repaired, as CS children lack CSA or CSB, the DNA repair genes. This may explain one of the most prevalent phenotypes in Cockayne Syndrome: a failure to thrive and cachexia.
The authors tested this theory on an ADH5-CSB mice model. A CSB mice model alone only has mild neurological symptoms of Cockayne Syndrome, but once the aldehyde (ADH5) was introduced, and GDF-15 began to be released, the mice developed severe symptoms of the disease. However, the good news was that an antibody to GDF-15, developed by Pfizer, was able to alleviate the symptoms in mice. Our study seeks to test this phenomenon in children, and determine, at a minimum, whether the GDF-15 levels are consistently elevated, and whether the Pfizer antibody to GDF-15 could potentially be safe and effective for children. All testing will be conducted at Mayo Clinic Laboratories.
For more information or if you are interested in working with us on this project, please contact us at info@riaanresearch.org.
Riaan Research Initiative is seeking a clinical partner to conduct a small and short study involving analyzing serum samples from children with Cockayne Syndrome. The study’s goal is to assess GDF-15 serum levels in a small number of children with Cockayne Syndrome and determine whether the levels are elevated, and if so, how elevated. This study is based on a recent paper that hypothesizes that GDF-15, a growth differentiation factor that tells the brain to reduce appetite and induces weight loss, may be perpetually released in Cockayne Syndrome children, causing adverse effects.
Researchers from Dr. KJ Patel’s lab discovered that when formaldehyde is introduced to the body, it causes DNA damage. The kidneys subsequently release GDF-15, which triggers appetite suppression while the body attempts to repair the DNA damage. This response is also commonly seen in patients undergoing chemotherapy. Scientists believe that the body may have developed this evolutionary appetite block to prevent a person from ingesting more toxins while it is repairing DNA damage. Researchers theorize that as DNA damage builds up in Cockayne Syndrome patients, GDF-15 continues to be released because the DNA damage is never repaired, as CS children lack CSA or CSB, the DNA repair genes. This may explain one of the most prevalent phenotypes in Cockayne Syndrome: a failure to thrive and cachexia.
The authors tested this theory on an ADH5-CSB mice model. A CSB mice model alone only has mild neurological symptoms of Cockayne Syndrome, but once the aldehyde (ADH5) was introduced, and GDF-15 began to be released, the mice developed severe symptoms of the disease. However, the good news was that an antibody to GDF-15, developed by Pfizer, was able to alleviate the symptoms in mice. Our study seeks to test this phenomenon in children, and determine, at a minimum, whether the GDF-15 levels are consistently elevated, and whether the Pfizer antibody to GDF-15 could potentially be safe and effective for children. All testing will be conducted at Mayo Clinic Laboratories.
For more information or if you are interested in working with us on this project, please contact us at info@riaanresearch.org. Understanding Cockayne Syndrome
For more on how Cockayne Syndrome impacts diagnosed children, we recommend reviewing the research papers below.
- Nance and Berry (1993): Cockayne Syndrome: Review of 140 cases
- Valerie Natale (2011): A Comprehensive Description of the Severity Groups in Cockayne Syndrome
- Masaya Kubota (2015): Nationwide survey of Cockayne Syndrome
- B.T. Wilson (2016): The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care
- Vincent Laugel (2021): Growth Charts in Cockayne Syndrome type 1 and 2
Cockayne Syndrome is a complex and lethal multi-system disorder representing a failure in DNA transcription and repair.
An important aspect of Cockayne Syndrome is the body’s inability to displace or remove RNA polymerase II – the enzyme that “reads” our genes – once it gets stuck by DNA damage. CSB is important for marking this enzyme for degradation, and researchers believe that RNA polymerase remains stalled in the absence of CSB (also implicated in the absence of CSA) and that this may explain some of the severe neurological features of the disease.
Gene replacement therapy remains one of the best treatment options for Cockayne Syndrome.
Riaan Research Initiative’s pilot project is to help support and fund research into gene replacement therapy, with a goal of safely and quickly translating it from the lab to the bedside, for genetic mutations in the CSA/ERCC8 gene.
Cockayne Syndrome, currently lacking any approved treatment, results in:
- Severe neurodegeneration
- Microcephaly
- Growth failure, including feeding problems
- Vision & hearing problems including cataracts
- Global developmental delays
- Limits or completely impairs a child's ability to sit, stand, walk, talk, and/or feed themselves
- Causes early death
Cockayne Syndrome Subtypes
Cockayne Syndrome is typically divided into three types for clinical purposes. However, researchers are moving away from this thinking and classifying it as a spectrum.
Type I
‣ Most common and a "moderate" version of the disease with children surviving into their late teens.
‣ Symptoms appear shortly after birth to a year or later.
Type II
‣ Most severe version of the disease with death ocurring typically by five years of age.
‣ Symptoms appear at birth or a bit later.
Type III
‣ A mild version of the disease with an average lifespan of 40 to 50 years.
‣ Symptoms appear later in childhood
In October 2021, Riaan Research Initiative announced launching the pre-clinical phase of the CSA gene replacement therapy program at the University of Massachusetts Chan Medical School.
CSA is ideal for gene replacement therapy because of its small size, allowing it to fit into adenovirus technology (AAV9 vector), which will be used to deliver a healthy copy of the gene into the human body.
Our goal is to carry the pre-clinical research and development of an AAV9 vector for CSA to a human clinical trial – which will cost up to four million dollars.

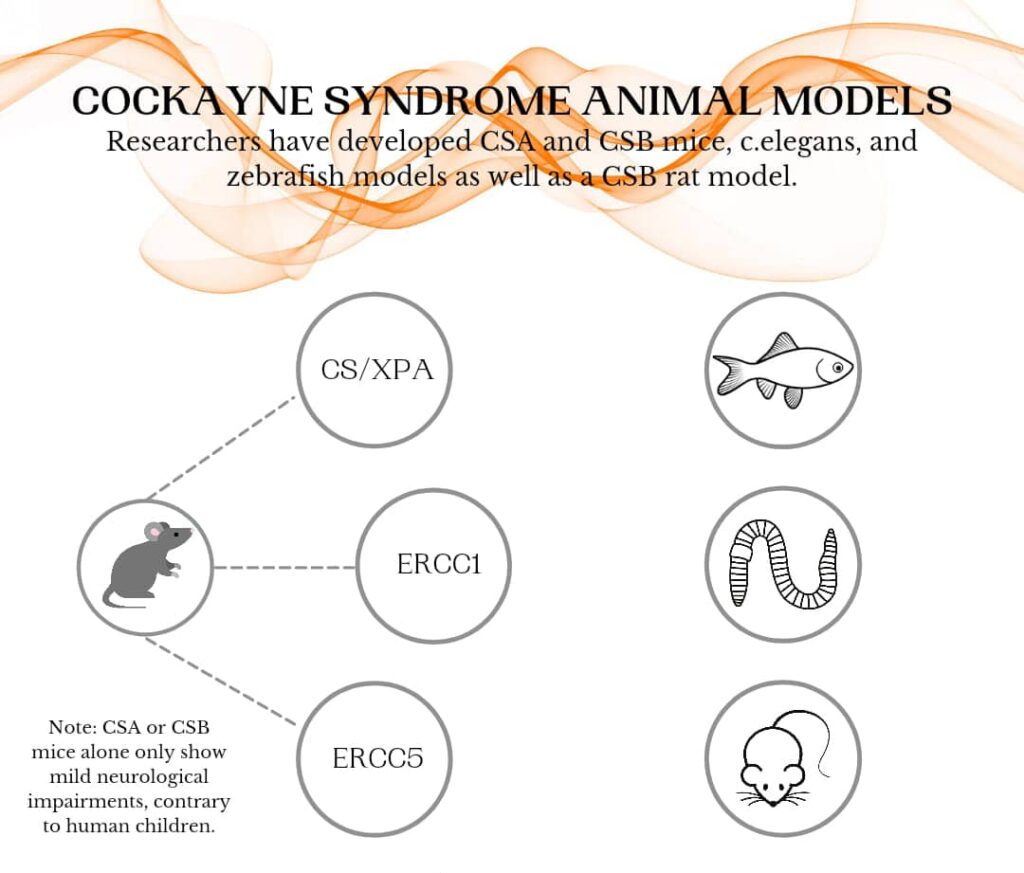
There are three identified Cockayne Syndrome mouse models that produce the severe neurological symptoms seen in humans: CSA/XPA or CSB/XPA mice; XPG (ERCC5) mice; and ERCC1 mice. Pre-clinical gene therapy studies have demonstrated good results in XPG mice. Data from ERCC1 mice is forthcoming.
Pre-clinical gene therapy studies on the CSA/XPA double knockout models are now underway at the University of Massachusetts Chan Medical School, funded by Riaan Research Initiative.
Animal Models for Cockayne Syndrome
CSA Gene Replacement Therapy Project
What is gene replacement therapy?
How does gene replacement therapy work?
How is gene therapy administered?
In the case of neurodegenerative conditions, the vector can be administered to the Central Nervous System via bilateral intrathalamic, intrathecal, intra-cisterna magna (ICM), or intracerebroventricular (ICV) delivery. It is usually a one-time treatment, but may require “a booster” years later.
(Source: Explore Gene Therapy)
Scientists create a healthy, functional copy of a mutated human gene, test to ensure it is safe, and administer the new gene to the body to allow it to make the protein it is lacking.
Scientists place the gene inside a vector, which is a vehicle for delivering the gene throughout the body. A viral vector such as an adeno-associated virus called AAV is preferable as a vehicle because the virus does not make people sick.
In the case of neurodegenerative conditions, the vector can be administered to the Central Nervous System via bilateral intrathalamic, intrathecal, intra-cisterna magna (ICM), or intracerebroventricular (ICV) delivery. It is usually a one-time treatment, but may require “a booster” years later.
(Source: Explore Gene Therapy)
The Gene Replacement Therapy Research and Development Process for CSA Mutations
We cannot administer a CSA gene replacement therapy to humans until we know it is effective (i.e. does it increase gene expression in mice or other smaller animals that also lack the CSA gene), determine correct dosing (we always want to give the lowest possible dose of a medication to children), and until we can prove that it is safe to use in babies and children.
In order to prove the adenovirus vector (AAV9) carrying a healthy CSA gene is effective and repairs the defects caused by the mutated CSA gene in the human body, we must fund research to engage in pre-clinical studies, which can include testing on mice with a double knock-out of the CSA and the XPA gene. Because the double knock-out CSA/XPA mice produce the severe Cockayne Syndrome phenotype seen in humans, they are ideal for testing a CSA gene therapy. Researchers have already administered gene therapy to mice with an ERCC5 mutation, which also produces a Cockayne Syndrome phenotype, with success. This demonstrates the possibilities for a human trial.
For more on CSA/XPA mice: Neurovascular dysfunction and neuroinflammation in a Cockayne Syndrome mouse model (2021).
Once we clear pre-clinical studies and establish proof of concept, the next step is to engage in toxicology studies to ensure the therapy is safe, manufacture a clinical-grade drug, and then apply for an Investigational New Drug (IND) application with the U.S. Food and Drug Administration to gain approval for a human trial.
Gene replacement therapy has the potential to cure mutations in the ERCC8/CSA gene, and improve Riaan’s quality and longevity of life, and that of other children and families impacted by this relentless and devastating disease. Our ability to demonstrate proof of concept utilizing gene replacement therapy for a complicated neurodegenerative disorder caused by mutations in ERCC8/CSA opens the door to curing genetic disease, period. Gene replacement therapy is the new era of science, the beginning of a revolutionary understanding of modern medicine and disease.
We ask you to help fund this innovative, exciting, and life-saving work and allow us to move forward and help transform therapies like this, and others, into safe treatments for babies and children.
The Research Team at the Horae Gene Therapy Center
University of Massachusetts Chan Medical School

Dr. Miguel Sena Esteves

Dr. Rita Batista
Riaan Research Initiative has partnered with the lab of Dr. Miguel Sena Esteves, PhD of the Li Weibo Institute for Rare Diseases Research at the University of Massachusetts Chan Medical School to launch the pre-clinical phase of the CSA/ERCC8 gene replacement therapy program. Dr. Sena Esteves will serve as co-Principal Investigator on the project, along with Dr. Rita Batista.
Dr. Sena Esteves’ laboratory develops novel AAV capsids to improve targeting, distribution, and potent transduction of target tissues in central nervous system diseases. He is part of a team of researchers and physicians that developed AAV gene therapies for GM2 gangliosidosis (Tay-Sachs and Sandhoff diseases), and GM1 gangliosidosis, both currently being tested in phase I/II clinical trials. Dr. Sena Esteves has authored 88+ peer-reviewed publications and is an inventor on several patent applications.
Dr. Rita Batista, PhD is an Instructor of Neurology at University of Massachusetts Chan Medical School, and is co-Principal Investigator on the CSA/ERCC8 gene replacement therapy research and development project. She has a background in Biochemistry and earned her PhD in Biomedical Sciences from University of Porto in Portugal, with her research focused in generating new somatic transgenic mouse models of transthyretin amyloidosis to better understand disease pathogenesis and develop novel gene therapies. Dr. Batista joined the Sena Esteves lab at UMass Chan in 2014 as a postdoc to develop new gene therapies for Huntington’s Disease, as well as to study different approaches for a better distribution of AAV viral vectors into the central nervous system. As she is paving her way to independence and establishing her own lab, Dr. Batista’s research interests focus on finding the mechanisms of rare neurological diseases and developing strategies for treatment using AAV gene therapy.
Other members of the CSA/ERCC8 gene replacement therapy team include international gene therapy pioneer and Dean of the University of Massachusetts Chan Medical School Dr. Terence Flotte, and Dr. Alisha Gruntman, who designed the first known rAAV-CSA vector.
🔎
The UMass team is currently seeking a Research Associate to join the CSA gene replacement therapy project.
Drug Repurposing Projects
Rarebase Function Project
In October 2021, Riaan Research Initiative partnered with Rarebase, a public biotech company based in Palo Alto, California to screen repurposed drugs, utilizing induced pluripotent stem cells as a disease model. Through the Function program, we hope to determine whether existing drugs may compensate for the lack of gene expression in patients with mutations in CSA/ERCC8. We anticipate results in mid to late 2022.
"We're thrilled to be collaborating with Riaan Research Initiative to help find treatments for patients with Cockayne Syndrome. There are thousands of approved drugs, and many of them can have applications beyond their routinely prescribed indication. If such treatments are out there for Cockayne Syndrome, we are committed to finding them using our Function platform," said Onno Faber, Co-founder and CEO of Rarebase.
Leiden University Medical Center and Charles River Laboratories
Riaan Research Initiative is partnering with Leiden University Medical Center and Charles River Laboratories to engage in the most comprehensive drug repurposing screen to date using an innovative and optimized Cockayne Syndrome specific-assay and patient fibroblasts. Specifically, our work will be focused on whether we can find drugs that can deactivate a gene that is triggered by failed transcription resulting from mutations in genes CSA or CSB, and is associated with neurodegenerative features linked to the Cockayne Syndrome phenotype, including neurosensory motor delays, and microcephaly.
Our hope is to find an already FDA-approved drug that may work to halt or reverse some of the neurodegeneration we see in Cockayne Syndrome patients.